 Solubility in Water Solubility in Water
 Water has been referred to as the "universal solvent", and its widespread distribution on this planet and essential role in life make it the benchmark for discussions of solubility. Water dissolves many ionic salts thanks to its high dielectric constant and ability to solvate ions. The former reduces the attraction between oppositely charged ions and the latter stabilizes the ions by binding to them and delocalizing charge density. Water has been referred to as the "universal solvent", and its widespread distribution on this planet and essential role in life make it the benchmark for discussions of solubility. Water dissolves many ionic salts thanks to its high dielectric constant and ability to solvate ions. The former reduces the attraction between oppositely charged ions and the latter stabilizes the ions by binding to them and delocalizing charge density.
 Many organic compounds, especially alkanes and other hydrocarbons, are nearly insoluble in water. Organic compounds that are water soluble, such as most of those listed in the table below, generally have hydrogen bond acceptor and donor groups. Many organic compounds, especially alkanes and other hydrocarbons, are nearly insoluble in water. Organic compounds that are water soluble, such as most of those listed in the table below, generally have hydrogen bond acceptor and donor groups.
 The least soluble of the listed compounds is diethyl ether, which can serve only as a hydrogen bond acceptor and is 75% hydrocarbon in nature. Even so, diethyl ether is about two hundred times more soluble in water than is pentane. The least soluble of the listed compounds is diethyl ether, which can serve only as a hydrogen bond acceptor and is 75% hydrocarbon in nature. Even so, diethyl ether is about two hundred times more soluble in water than is pentane.
 The chief characteristic of water that influences these solubilities is the extensive hydrogen bonded association of its molecules with each other. This hydrogen bonded network is stabilized by the sum of all the hydrogen bond energies, and if nonpolar molecules such as hexane were inserted into the network they would destroy local structure without contributing any hydrogen bonds of their own. The chief characteristic of water that influences these solubilities is the extensive hydrogen bonded association of its molecules with each other. This hydrogen bonded network is stabilized by the sum of all the hydrogen bond energies, and if nonpolar molecules such as hexane were inserted into the network they would destroy local structure without contributing any hydrogen bonds of their own.
 Of course, hexane molecules experience significant van der Waals attraction to neighboring molecules, but these attractive forces are much weaker than the hydrogen bond. Consequently, when hexane or other nonpolar compounds are mixed with water, the strong association forces of the water network exclude the nonpolar molecules, which must then exist in a separate phase. This is shown in the following illustration, and since hexane is less dense than water, the hexane phase floats on the water phase. Of course, hexane molecules experience significant van der Waals attraction to neighboring molecules, but these attractive forces are much weaker than the hydrogen bond. Consequently, when hexane or other nonpolar compounds are mixed with water, the strong association forces of the water network exclude the nonpolar molecules, which must then exist in a separate phase. This is shown in the following illustration, and since hexane is less dense than water, the hexane phase floats on the water phase.
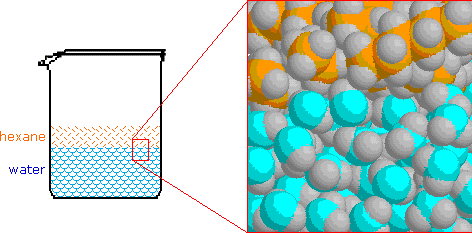
 It is important to remember this tendency of water to exclude nonpolar molecules and groups, since it is a factor in the structure and behavior of many complex molecular systems. A common nomenclature used to describe molecules and regions within molecules is hydrophilic for polar, hydrogen bonding moieties and hydrophobic for nonpolar species. It is important to remember this tendency of water to exclude nonpolar molecules and groups, since it is a factor in the structure and behavior of many complex molecular systems. A common nomenclature used to describe molecules and regions within molecules is hydrophilic for polar, hydrogen bonding moieties and hydrophobic for nonpolar species.
Virtual Textbookの目次へ |